OKR template to secure FDA approval for the capital equipment medical device
Your OKR template
The second objective looks toward obtaining confirmation of FDA approval by the closure of the quarter. Steps consist of regularly following up with the FDA, preparing and submitting all required documents, and adhering to all response deadlines to any FDA inquiries without fail.
The third significant objective is to pass all necessary FDA inspections without any citations. This will involve introducing strict internal quality control measures, carrying out regular inspections to maintain compliance, and understanding all FDA guidelines and expectations.
By successfully accomplishing these objectives, the company would efficiently expedite the approval process of the crucial capital equipment medical device and secure its clearance through FDA within the quarter.
ObjectiveSecure FDA approval for the capital equipment medical device
KRComplete and submit comprehensive FDA application by week 4
Thoroughly fill out the FDA application form accurately and comprehensively
Gather all necessary documentation and information for FDA application
Submit the completed FDA application before the deadline in week 4
KRObtain confirmation of FDA approval by end of the quarter
Regularly follow-up with FDA regarding application status
Prepare and submit all necessary documentation for FDA approval
Ensure all response deadlines to FDA inquiries are met
KRPass all necessary FDA inspections successfully with zero citations
Implement strict internal quality control measures
Regularly inspect and document all processes for compliance
Review and comprehend all FDA guidelines and expectations
How to edit and track OKRs with Tability
You'll probably want to edit the examples in this post, and Tability is the perfect tool for it.
Tability is an AI-powered platform that helps teams set better goals, monitor execution, and get help to achieve their objectives faster.
With Tability you can:
- Use AI to draft a complete set of OKRs in seconds
- Connect your OKRs and team goals to your project
- Automate reporting with integrations and built-in dashboard
Instead of having to copy the content of the OKR examples in a doc or spreadsheet, you can use Tability’s magic importer to start using any of the examples in this page.
The import process can be done in seconds, allowing you to edit OKRs directly in a platform that knows how to manage and track goals.
Step 1. Sign up for a free Tability account
Go tohttps://tability.app/signup and create your account (it's free!)
Step 2. Create a plan
Follow the steps after your onboarding to create your first plan, you should get to a page that looks like the picture below.
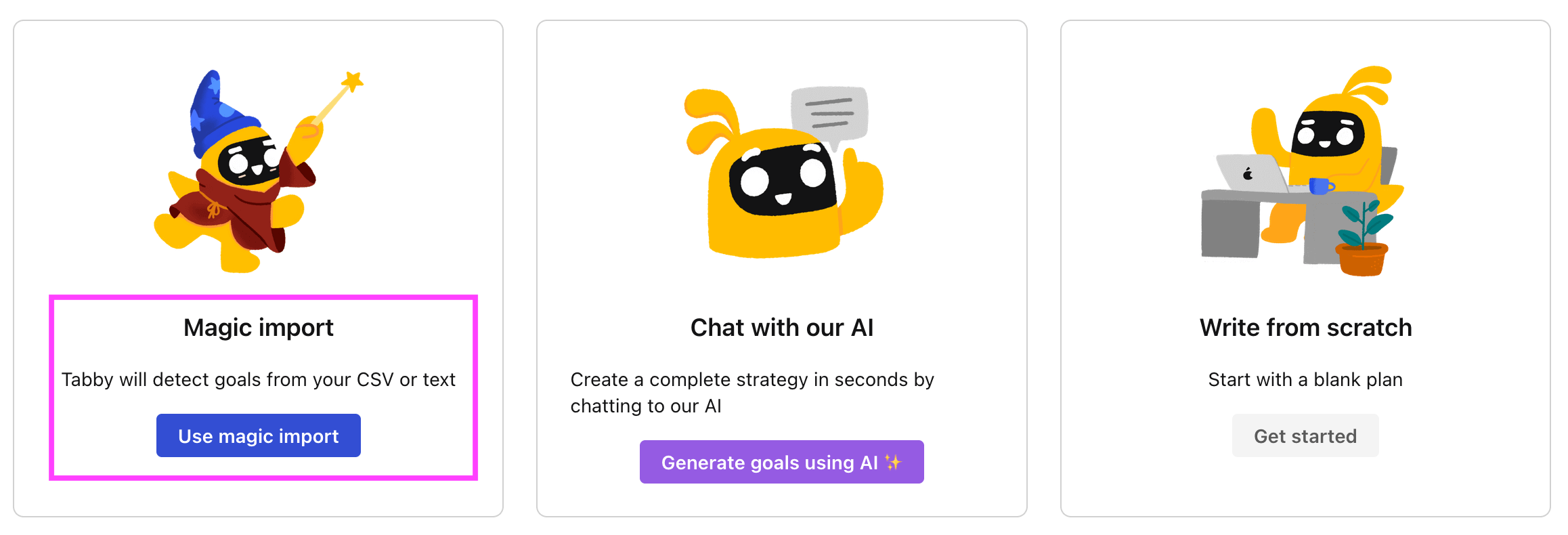
Step 3. Use the magic importer
Click on Use magic import to open up the Magic Import modal.
Now, go back to the OKR examples, and click on Copy on the example that you’d like to use.

Paste the content in the text import section. Don’t worry about the formatting, Tability’s AI will be able to parse it!

Now, just click on Import from text and let the magic happen.
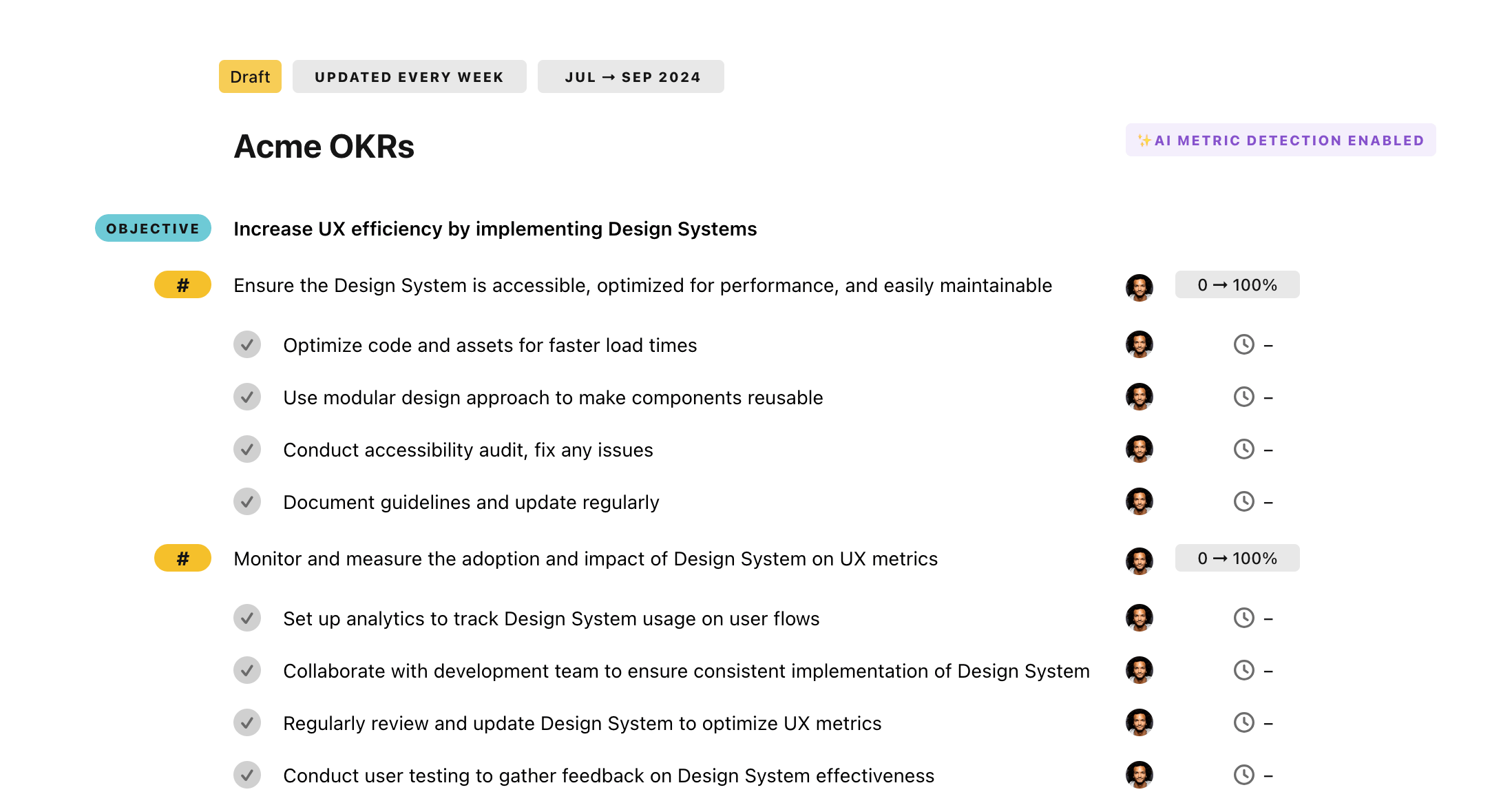
Once your example is in the plan editor, you will be able to:
- Edit the objectives, key results, and tasks
- Click on the target 0 → 100% to set better target
- Use the tips and the AI to refine your goals
Step 4. Publish your plan
Once you’re done editing, you can publish your plan to switch to the goal-tracking mode.
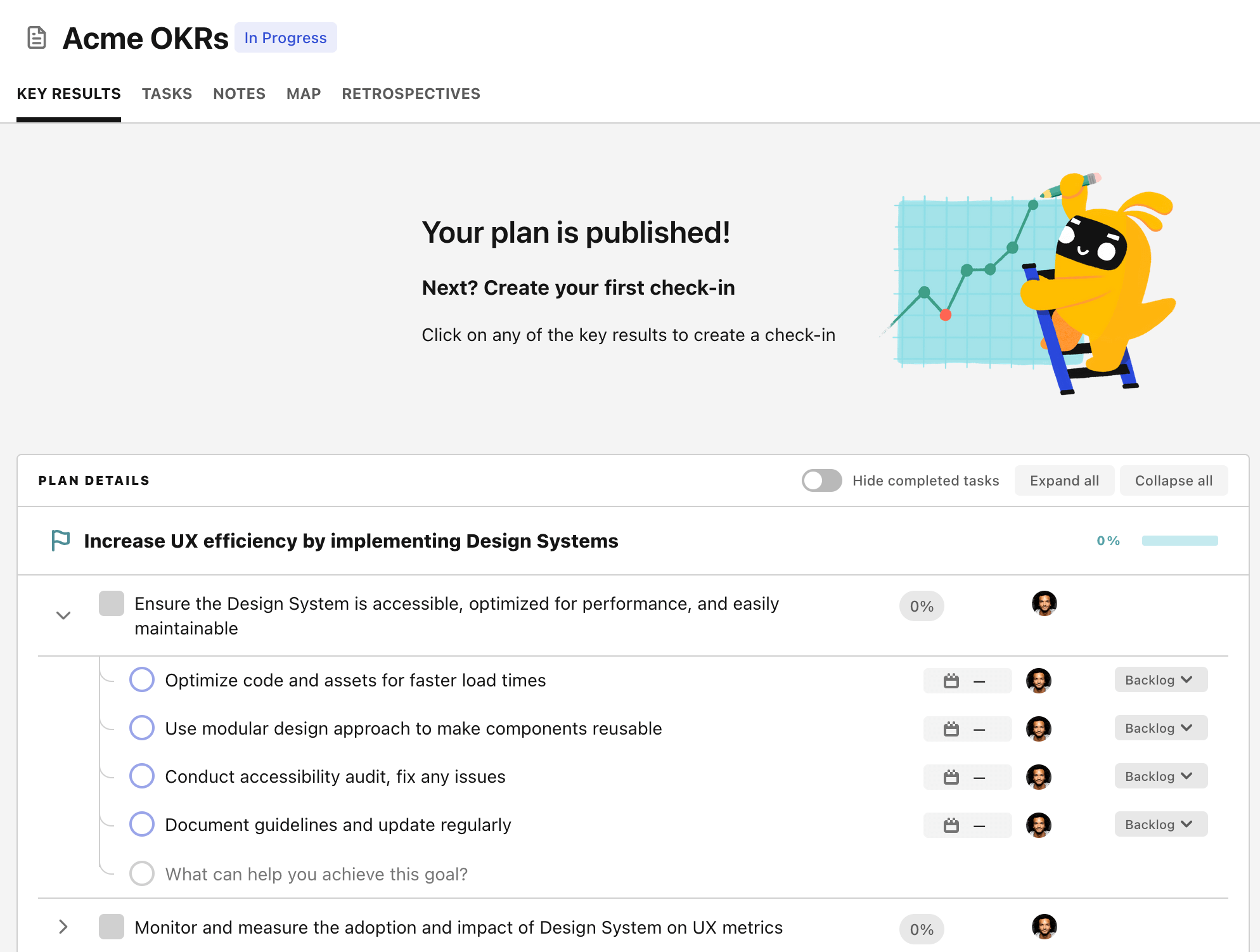
From there you will have access to all the features that will help you and your team save hours with OKR reporting.
- 10+ built-in dashboards to visualise progress on your goals
- Weekly reminders, data connectors, and smart notifications
- 9 views to map OKRs to strategic projects
- Strategy map to align teams at scale