Tability is a cheatcode for goal-driven teams. Set perfect OKRs with AI, stay focused on the work that matters.
What are Quality Compliance Officer OKRs?
The Objective and Key Results (OKR) framework is a simple goal-setting methodology that was introduced at Intel by Andy Grove in the 70s. It became popular after John Doerr introduced it to Google in the 90s, and it's now used by teams of all sizes to set and track ambitious goals at scale.
How you write your OKRs can make a huge difference on the impact that your team will have at the end of the quarter. But, it's not always easy to write a quarterly plan that focuses on outcomes instead of projects.
We have curated a selection of OKR examples specifically for Quality Compliance Officer to assist you. Feel free to explore the templates below for inspiration in setting your own goals.
If you want to learn more about the framework, you can read our OKR guide online.
The best tools for writing perfect Quality Compliance Officer OKRs
Here are 2 tools that can help you draft your OKRs in no time.
Tability AI: to generate OKRs based on a prompt
Tability AI allows you to describe your goals in a prompt, and generate a fully editable OKR template in seconds.
- 1. Create a Tability account
- 2. Click on the Generate goals using AI
- 3. Describe your goals in a prompt
- 4. Get your fully editable OKR template
- 5. Publish to start tracking progress and get automated OKR dashboards
Watch the video below to see it in action 👇
Tability Feedback: to improve existing OKRs
You can use Tability's AI feedback to improve your OKRs if you already have existing goals.
- 1. Create your Tability account
- 2. Add your existing OKRs (you can import them from a spreadsheet)
- 3. Click on Generate analysis
- 4. Review the suggestions and decide to accept or dismiss them
- 5. Publish to start tracking progress and get automated OKR dashboards
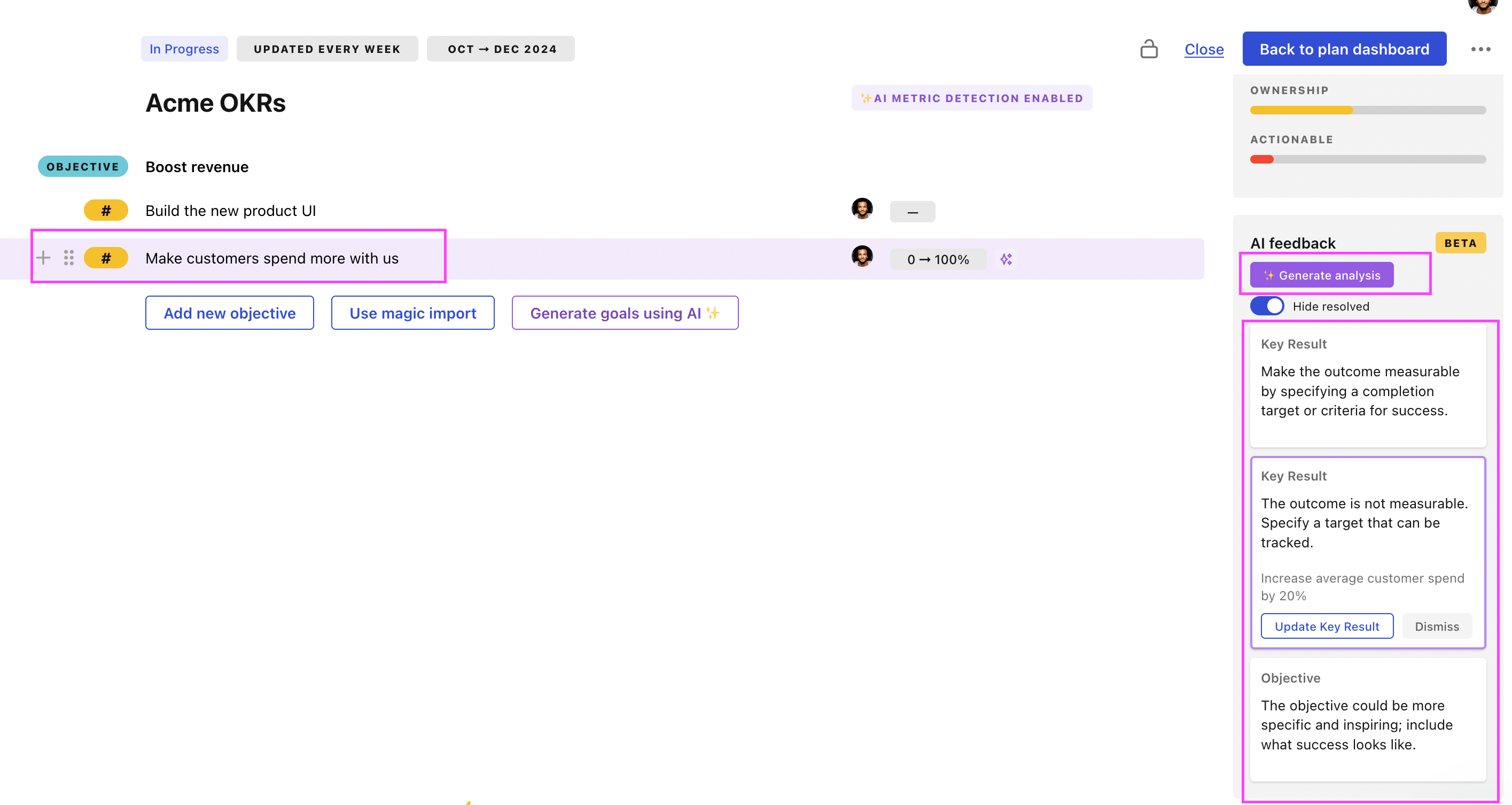
Tability will scan your OKRs and offer different suggestions to improve them. This can range from a small rewrite of a statement to make it clearer to a complete rewrite of the entire OKR.
Quality Compliance Officer OKRs examples
We've added many examples of Quality Compliance Officer Objectives and Key Results, but we did not stop there. Understanding the difference between OKRs and projects is important, so we also added examples of strategic initiatives that relate to the OKRs.
Hope you'll find this helpful!
OKRs to enhance the quality and regulatory compliance of debt collection practices
ObjectiveEnhance the quality and regulatory compliance of debt collection practices
KRComplete 100% of mandatory compliance trainings for all team members
Monitor and track team members' training progress
Set deadlines for completing each training course
Identify all mandatory compliance trainings for each team member
KRImplement a 15% improvement in quality assurance scores from customer feedback
Analyze customer feedback and identify areas needing improvement
Train staff on identified areas to rectify issues
Implement customer-directed quality assurance initiatives
KRReduce non-compliance issues by 20% through periodic audits and refinements
Establish process for identifying and correcting non-compliance
Implement follow-up reviews to confirm resolutions
Develop a schedule for regular compliance audits
OKRs to streamline policy and clinical documentation variations
ObjectiveStreamline policy and clinical documentation variations
KRConduct thorough audits on 80% of existing policy and clinical documentation
Create strategic plan for selecting policies to audit
Start the documentation auditing process
Train auditing team on documentation appraisal
KRImplement upgrades or improvements in 70% of identified discrepancy areas
Develop plan for implementing necessary upgrades
Prioritize identified discrepancies based on impact
Execute improvements in selected discrepancy areas
KRIncrease compliance in documentation alignment by 50% through targeted staff training
Develop focused training programs on documentation compliance
Provide feedback and ongoing support for improved alignment
Implement regular compliance checks on staff documentation
OKRs to achieve 100% compliance with relevant industry regulations and standards
ObjectiveEnsure regulatory compliance in all operations
KREstablish a system for continuous monitoring and reporting of compliance status
Define metrics to track compliance
Schedule regular compliance audits
Establish communication protocol for reporting
Design compliance reporting dashboard
KRAchieve 100% compliance with all relevant regulations and standards
Stay up-to-date with changes to regulations and standards
Conduct regular audits to ensure compliance
Provide ongoing training to employees
Implement measures to address non-compliance
KRDevelop and implement an updated compliance training program for all employees
Launch new compliance training program online and in-person
Evaluate effectiveness of updated program through employee feedback and assessments
Conduct a needs assessment to identify compliance training gaps
Create new, engaging training materials for all job functions
KRConduct a full audit of all operations and identify areas of regulatory risk
Identify gaps in compliance procedures and create action plan
Review each operation for compliance risks
Conduct training on regulations and compliance procedures
Evaluate existing controls and their effectiveness
OKRs to achieve substantial decrease in failed attestations and redemptions
ObjectiveAchieve substantial decrease in failed attestations and redemptions
KRLower redemption failures by 15% through improved risk management procedures
Implement robust quality assurance checks for redemptions
Conduct regular training on risk management protocols
Review and revise existing risk management procedures
KRImplement security compliance training for 80% of the team to minimize error rate
Schedule training for 80% of the team
Monitor and evaluate training effectiveness to reduce errors
Identify appropriate security compliance training program
KRReduce failed attestations by 20% with rigorous compliance checks
Regularly review and update check procedures
Implement stricter compliance check procedures
Train staff on compliance standard expectations
OKRs to secure FDA approval for our new pharmaceutical product
ObjectiveSecure FDA approval for our new pharmaceutical product
KRResolve all FDA queries or issues regarding the application within six weeks
Research and compile thorough responses to each issue
Submit all responses and corrections to FDA within six weeks
Identify all FDA queries or issues on the application
KRSubmit a complete and compliant application to FDA within the first month
Review FDA guidelines to ensure application compliance
Submit the completed application to the FDA
Gather all necessary documents and data for application
KRSuccessfully pass the FDA's inspection and audit of our production facilities
Ensure all documentation and records are accurate, updated, and easily accessible
Provide thorough training to staff on FDA regulations and requirements
Maintain the facility's cleanliness and safety according to FDA standards
Quality Compliance Officer OKR best practices
Generally speaking, your objectives should be ambitious yet achievable, and your key results should be measurable and time-bound (using the SMART framework can be helpful). It is also recommended to list strategic initiatives under your key results, as it'll help you avoid the common mistake of listing projects in your KRs.
Here are a couple of best practices extracted from our OKR implementation guide 👇
Tip #1: Limit the number of key results
Having too many OKRs is the #1 mistake that teams make when adopting the framework. The problem with tracking too many competing goals is that it will be hard for your team to know what really matters.
We recommend having 3-4 objectives, and 3-4 key results per objective. A platform like Tability can run audits on your data to help you identify the plans that have too many goals.
Tip #2: Commit to weekly OKR check-ins
Setting good goals can be challenging, but without regular check-ins, your team will struggle to make progress. We recommend that you track your OKRs weekly to get the full benefits from the framework.
Being able to see trends for your key results will also keep yourself honest.
Tip #3: No more than 2 yellow statuses in a row
Yes, this is another tip for goal-tracking instead of goal-setting (but you'll get plenty of OKR examples above). But, once you have your goals defined, it will be your ability to keep the right sense of urgency that will make the difference.
As a rule of thumb, it's best to avoid having more than 2 yellow/at risk statuses in a row.
Make a call on the 3rd update. You should be either back on track, or off track. This sounds harsh but it's the best way to signal risks early enough to fix things.
Save hours with automated Quality Compliance Officer OKR dashboards
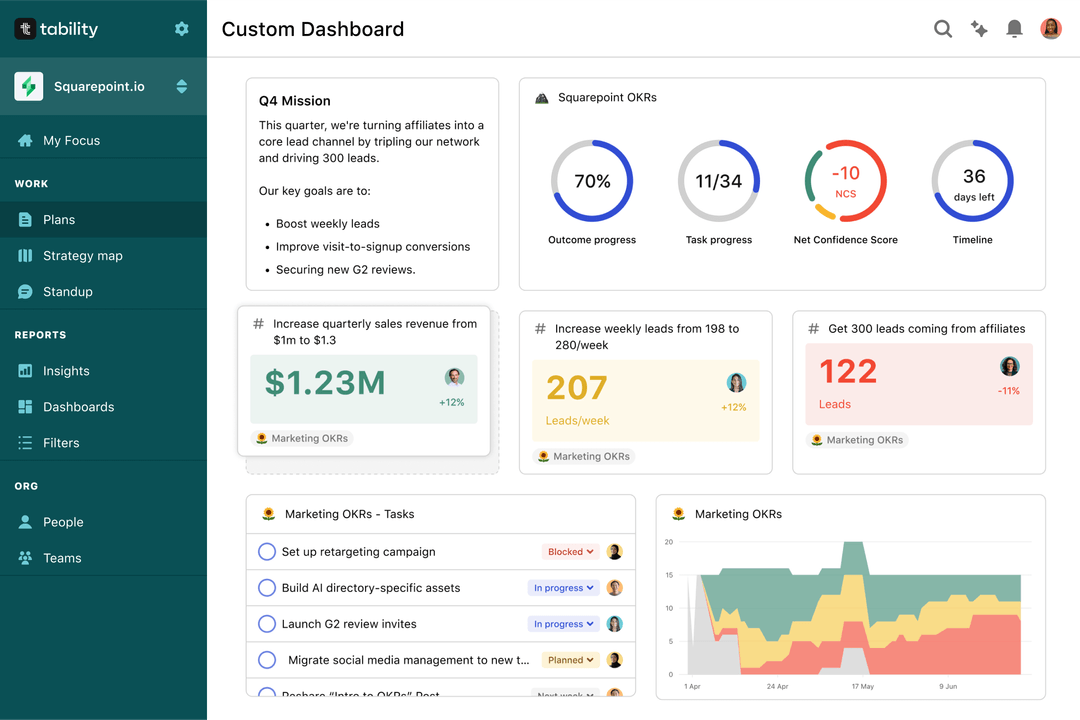
OKRs without regular progress updates are just KPIs. You'll need to update progress on your OKRs every week to get the full benefits from the framework. Reviewing progress periodically has several advantages:
- It brings the goals back to the top of the mind
- It will highlight poorly set OKRs
- It will surface execution risks
- It improves transparency and accountability
Spreadsheets are enough to get started. Then, once you need to scale you can use Tability to save time with automated OKR dashboards, data connectors, and actionable insights.
How to get Tability dashboards:
- 1. Create a Tability account
- 2. Use the importers to add your OKRs (works with any spreadsheet or doc)
- 3. Publish your OKR plan
That's it! Tability will instantly get access to 10+ dashboards to monitor progress, visualise trends, and identify risks early.
More Quality Compliance Officer OKR templates
We have more templates to help you draft your team goals and OKRs.
OKRs to enhance administrative efficiency and streamline daily operations
OKRs to improve overall teaching effectiveness for professors and lecturers
OKRs to enhance inpatient coordination and manage workload effectively
OKRs to determine sustainable funding requirements for existing programs
OKRs to increase overall team performance
OKRs to improve efficiency in meeting accounting deadlines